Biorepository
State-of-the-Art Infrastructure

Biorepository is housed in an area of 2766 sq feet. All the stored biospecimens are coded with alphanumeric codes and the participants IDs are de-identified by the use of Unique Indentification code (UIC)
Resources Available
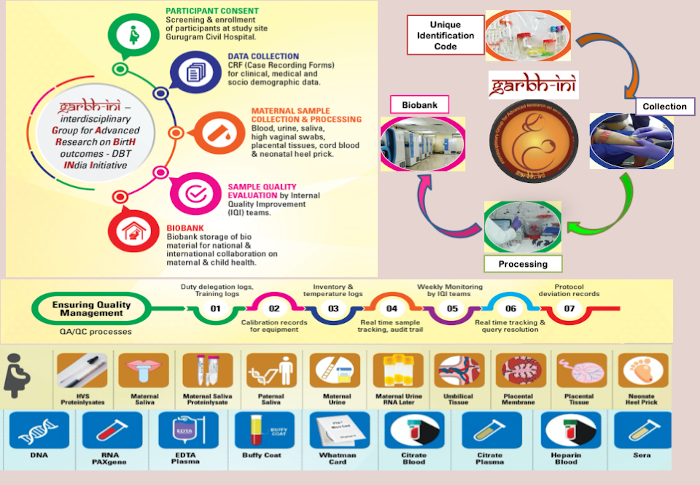
Currently, Biorepository is storing ~ 800,000 biospecimens from Garbh-Ini study cohort which include maternal serum, blood, plasma, DNA, blood as PAX gene tubes, saliva, urine, high vaginal swabs, feces, cord blood, umbilical cord tissue, placental tissue punches, placental membranes, paternal saliva and neonatal heel prick venous blood that are being collected across pregnancy, at delivery and post- delivery
Validated & Customized Lab processes
Biorepository at THSTI assures standardized sample collection and storage of high quality biospecimens with barcoding facility according to international guidelines (ISBER-International Society for Biologicals and Environmental Biorepository).
Data Management Centre
The Data Management Centre (DMC) at THSTI provides comprehensive data management support for both intramural and extramural clinical research projects. With expertise in handling large cohorts and multicenter clinical trials, the DMC ensures high-quality data management throughout the study lifecycle, from data capture planning to the delivery of accurate, analysis-ready datasets.
Team
The DMC is powered by a dedicated core team comprising data scientists, programmers, data managers, and data entry operators. With extensive experience in data management, the team develops and maintains robust systems for data collection, processing, and analysis. Their expertise extends to Android, web, and desktop applications, along with proficiency in R, Python, and STATA, enhancing efficiency and accessibility in data management.
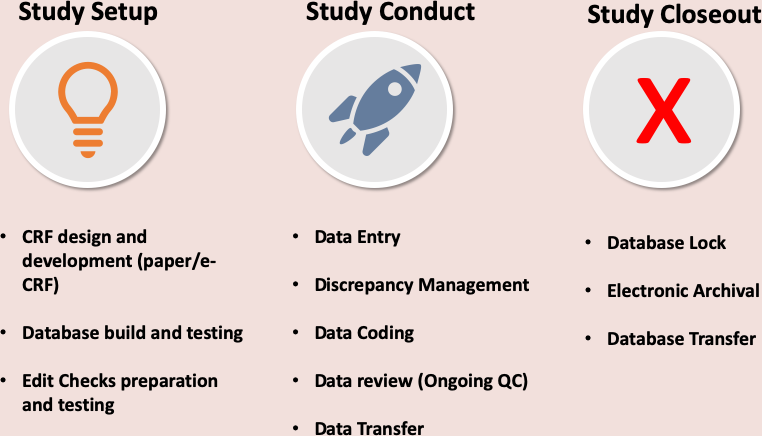
Data Management process for Paper Data Capture
The DMC supports both paper-based and electronic Case Report Forms (eCRFs) for data collection. The in-house Clinical Data Management Systems (CDMS) are robust, validated, and equipped with audit trails to ensure data integrity and reliability. These systems operate within a secure IT infrastructure and offer customized data management solutions, including:
- Development of data management plans
- CRF design and annotation
- Preparation of study-related technical documents, such as data validation plans
- Database development for electronic data capture
- Standard Operating Procedures (SOPs) for quality assurance
- Customized CRF filling guidelines
- Serious Adverse Event (SAE) data reconciliation for clinical trials
- Data import setup from third-party sources (e.g., central laboratory, imaging data)
- Generation of customized reports
- Procedures for data sharing, database locking prior to analysis, and data archiving

Data Management process for Electronic Data Capture
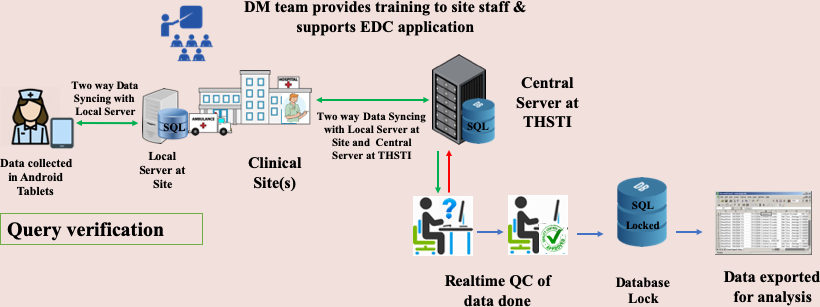
Data Security and Storage Ensuring data security, integrity, and confidentiality is a top priority at the DMC. The CDMS, Scheduler, and LMS employ a robust security framework that includes:
- Restricted user access with login credentials
- User activity monitoring
- Time-stamped audit trails to track data modifications
- De-identification of collected data using unique identification codes
- Controlled physical access to the DMC and data archival space
Aryabhata Datascience and Artificialintelligence Program at THSTI (ADAPT)
Key Objectives